ocrevus patient start form
Start infusion at 30mL per. The patient is currently on another.
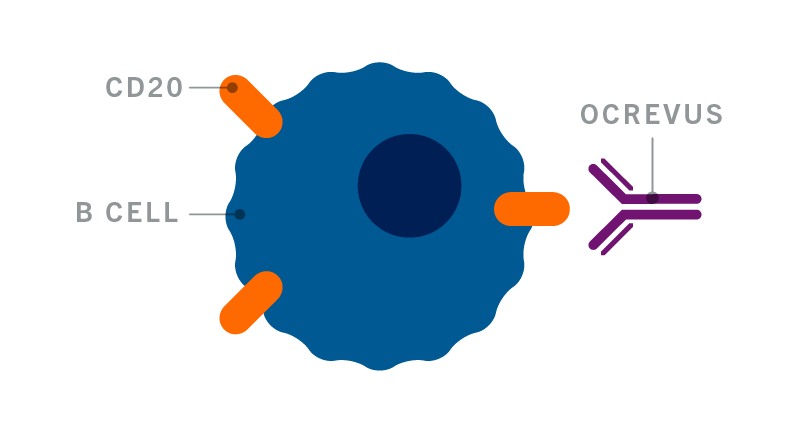
Ocrevus Access Solutions Patients And Caregivers
It must be completed by the provider.
. This is an optional form that can be used to provide information to your patients infusion site. Prescription Enrollment Form Ocrevus. If your patient has already begun treatment with drug samples of Ocrevus please choose new start of therapy.
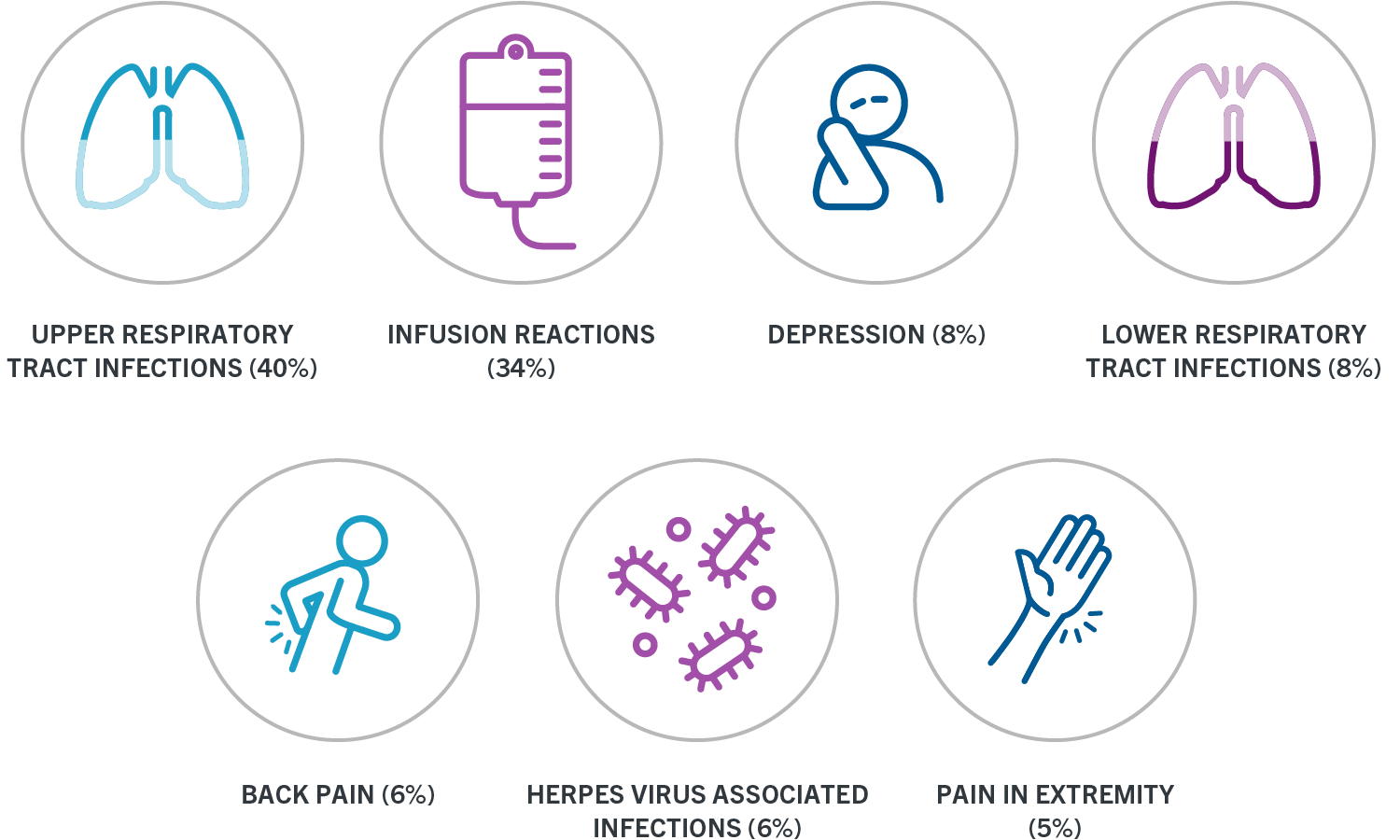
The OCREVUS Start Form is required for enrollment in OCREVUS Access Solutions. OCREVUS if a life-threatening or disabling infusion reaction occurs23 51 Infections. These infusion reactions can happen for up to 24 hours after your infusion.
Physicians are encouraged to. Ocrevus ocrelizumab Fax completed form to 8883021028. New patient Current patient Patients first name Last name Middle initial Male Female Last 4 digits of SSN Date of birth.
Patients first name. Genentech can start helping you when page 4 of this form is submitted by you or your doctors office in one of the following ways. New start of therapy continued therapy.
Please send this completed form to the infusion site of your choice not to Genentech. Prescription Enrollment Form. The form includes patient insurance and.
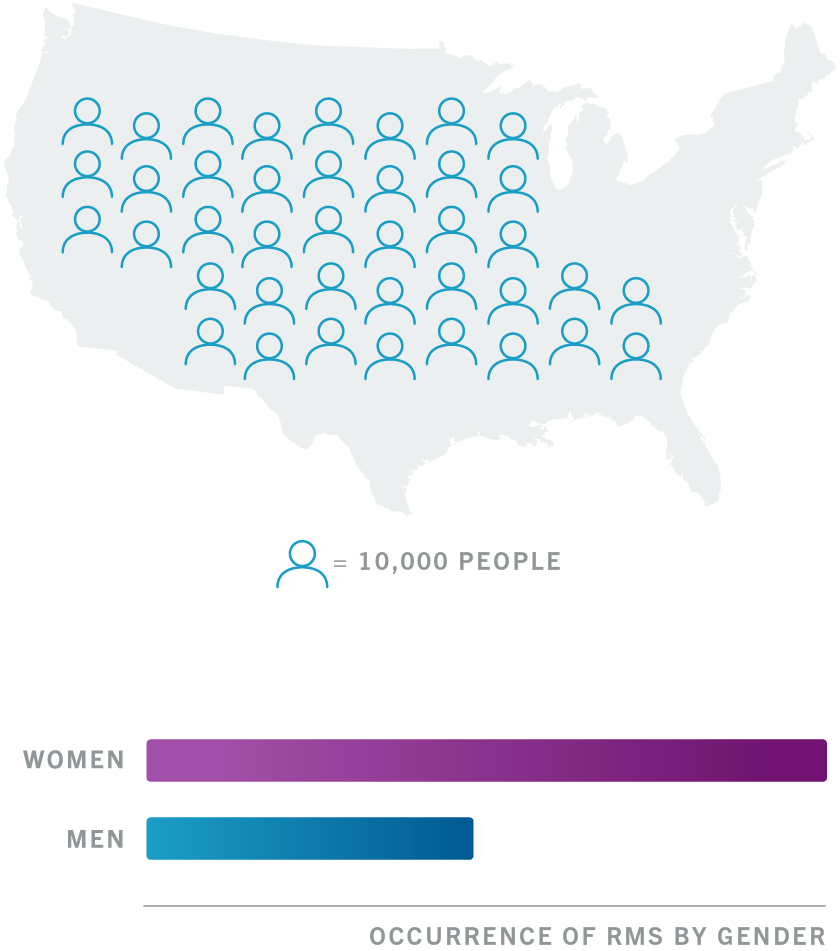
OCREVUS Start Form for ocrelizumab Who May See and Use My PII I authorize Genentech andor Genentech Patient Foundation to i use my PII for the purpose of facilitating my access. Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and active secondary. Swelling of the throat.
A representative from OCREVUS Access Solutions or your. Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and active secondary. OCREVUS is a prescription medicine used to treat.
Delay OCREVUS administration in patients with an active infection until the infection is resolved. There is a pregnancy exposure registry that monitors pregnancy and fetalneonatalinfant outcomes in women exposed to OCREVUS during pregnancy. It is important that.
OCREVUS is a prescription medicine used to treat. Prescribers first name. Date of birth.

Genentech Ocrevus Ocrelizumab Information For Patients

Ocrevus After One Year On The Market What Do We Know Now Everyday Health

New Drug For Severe Form Of Ms Generates Glimmer Of Hope
Benefits Investigations Prior Authorization Resources Ocrevus Access Solutions

Pdf Health And Economic Impact Of Relapsing Forms Of Multiple Sclerosis In Greece The Storms Study
Ocrevus Access Solutions Patients And Caregivers

Efficacy And Safety Of Teriflunomide In Asian Patients With Relapsing Forms Of Multiple Sclerosis A Subgroup Analysis Of The Phase 3 Tower Study Journal Of Clinical Neuroscience

Should I Try This Amazing New Drug For Multiple Sclerosis Shots Health News Npr

Pdf Ocrelizumab Versus Placebo In Primary Progressive Multiple Sclerosis

Fillable Online Infusion Checklist For Ocrevus In Relapsing Or Fax Email Print Pdffiller

Eu Approves Roche S Ocrevus For Relapsing Forms Of Ms Drug Discovery And Development

Fda Approves Ocrevus As First Treatment For Relapsing Primary Progressive Ms

Ocrevus Start Form Pdf Fill Online Printable Fillable Blank Pdffiller
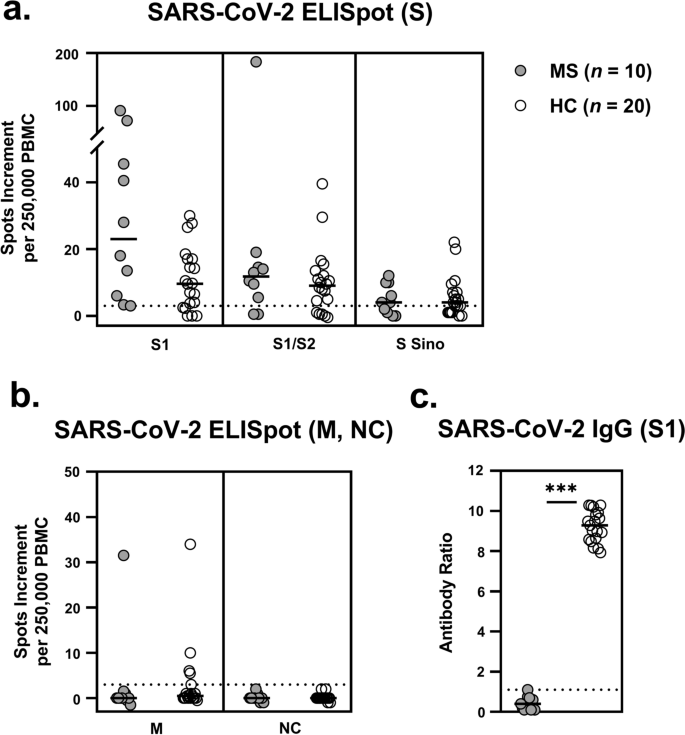
Evidence Of Extensive Cellular Immune Response After Sars Cov 2 Vaccination In Ocrelizumab Treated Patients With Multiple Sclerosis Neurological Research And Practice Full Text

Evolution Forms Of Multiple Sclerosis Ms A Primary Progressive Download Scientific Diagram